Custom Electronic Laboratory Notebook (ELN) Software
Capabilities, Development Plan, and Costs
In healthcare IT since 2005, ScienceSoft delivers compliant laboratory solutions that prioritize staff convenience and integrate easily with existing legacy systems.
Electronic Lab Notebook Software Essence
Electronic laboratory notebook (ELN) software digitizes and automates research activities in a laboratory setting, including research planning, execution, documenting research work, and reporting. It facilitates collaboration between researchers by providing them with a unified workspace and in-built collaboration tools.
A recent survey shows that 49% of R&D labs in medical research and the biotech or pharma industries view ELN implementation as the most urgent and important tech advancement.
Custom electronic lab notebook software can help labs overcome the challenges they often face with off-the-shelf ELN software, such as limited integration capabilities, and a lack of functionality needed for specific research workflows or compliance controls.
For example:
- Pharma and biotech labs can implement strict controls to meet FDA 21 CFR Part 11 and HIPAA requirements for data traceability through features like audit trails and e-signatures.
- Toxicology labs, in addition to compliance controls, can also get custom data entry forms for toxicology experiments.
- Genomics labs can integrate an ELN with specialized bioinformatics tools, databases, and platforms, as well as incorporate features for processing large-scale, complex genomic data and ensuring accurate version control.
Implementation time: 6 to 16+ months.
Important integrations for an ELN: lab devices, a lab information management system (LIMS), inventory and asset management systems, an organization’s data warehouse or a data lake, partner systems (CROs, CDMOs, pharma industry partners), external research tools and databases.
Costs: $120,000–$600,000, depending on the feature set. Answer a few questions about your business needs to get a custom quote from our consultants.
Capabilities of an Electronic Laboratory Notebook (ELN)
Core capabilities
Complementary modules
The following modules can be incorporated into an ELN solution or be a part of other laboratory software, such as laboratory information management system (LIMS), inventory and asset management systems, and LES software.
Important Integrations for an Electronic Lab Notebook
Below is the outline of ELN integrations ScienceSoft’s consultants consider the most valuable for enhancing research efficiency and a laboratory’s business performance. However, the integration plan may vary depending on each organization’s workflows and existing software.

- LIMS, inventory and asset management solutions — to connect sample tracking data with the experiments associated with it; to automatically update inventory levels based on experiment usage logged in the ELN; to check reagent availability during the experiment planning stage; to facilitate collaboration between operational and research teams.
- Laboratory devices (e.g., centrifuges, pipetting systems, chromatographs, DNA sequencers) — to enable automatic test result capture and test report generation.
- Laboratory execution system (LES) - to share instructions for executing SOPs and experimental lab procedures with lab operators and oversee their adherence.
- Scientific Data Management System (SDMS) and the organization’s data warehouse or lake - to export findings to a centralized repository; to utilize advanced cloud-based analytics for scientific discoveries.
- Clinical trial management system (CTMS) - to allow principal investigators (PIs) to oversee lab experiments; to link preclinical findings with clinical trial progress.
- Research and collaboration tools, external resources - to access external scientific tools (e.g., drawing tools for chemical compounds, tools for statistical analysis) and scientific literature databases directly from the ELN.
- Partners’ systems - to simplify the sharing of research data and orders between the lab and CROs, CDMOs, or partnering pharmaceutical companies.
How to Develop Electronic Lab Notebook Software
Custom development enables laboratories to get an ELN solution that can be integrated with any lab devices or existing legacy systems and includes role-specific workflows unique to the organization.
At the same time, it provides all essential features for software compliance with applicable standards and regulations. They may include FDA 21 CFR Part 11, GLP, OECD Principles of GLP, patient data privacy and security regulations such as HIPAA, GDPR (for labs dealing with patient data), GCP, EUDRALEX Annex 11 (for labs involved in clinical trials), GMP, and PIC/S (for pharmaceutical R&D labs).
1.
Business analysis and requirement gathering
At this step, business analysts study the laboratory’s existing software and interview the stakeholders (CIO, researchers, lab managers) to understand what goals should be achieved with the help of ELN. Based on the gathered information, they define the scope of operations custom ELN software will cover.
For example, a small academic lab may need a solution focusing solely on experimental planning and rule-based test result processing. Avoiding unnecessary costly features would help the academic laboratory reduce the solution’s TCO while getting all expected ELN benefits. Conversely, a large-scale biotech research company may benefit from advanced features like automated sample labeling and barcoding, microorganism collection management, and AI-powered analytics.
To solidify the solution vision, business analysts determine user roles (e.g., researchers, lab operators) that will use the ELN and list tasks each role needs to complete in the software. At the same time, business analysts define a compliance framework and ELN features that will help to achieve software compliance.
For instance, to support GLP and FDA 21 CFR Part 11 compliance, custom ELN software needs security controls (role-based access control, multi-factor authentication, and data encryption at rest and in transit), traceability features (e.g., version control, audit trails, and time-stamped electronic records), integrated device authenticity checks, a conversion tool for supplying copies of electronic record copies in portable formats, etc.
2.
Architecture design and integration planning
In collaboration with business analysts and the laboratory’s IT team, software architects determine integration methods for instruments and key laboratory software (e.g., LIMS, LES) and define the optimal way to incorporate the ELN in the organization’s existing data structure. When legacy challenges arise, architects propose strategies to preserve ELN compatibility with existing systems and data models while meeting current standards for interoperability. For instance, they may suggest developing custom APIs or conducting pinpoint modernization of legacy systems.
The architects and the lab’s IT team also decide on suitable ELN hosting options, such as selecting a cloud provider or opting for on-premises deployment.
3.
UX/UI design
UX designers create solution workflows tailored to the needs of specific user roles. For instance, lab technicians can benefit from task-focused dashboards and simplified data entry forms. On the other hand, researchers may need sophisticated data visualization tools to create interactive data graphs and an intuitive drag-and-drop interface to create multi-step workflows. For every scenario, designers ensure that frequently used tools are on the main screen while secondary ones are kept out of the way to avoid clutter and improve navigation.
4.
Development, QA, and deployment
At this stage, developers use the software requirements specification and UX/UI designs to code the back and front end of the ELN solution. ScienceSoft’s team recommends following the iterative approach to development, meaning that features are built and tested incrementally.
With this approach, as soon as the solution’s minimum viable version is delivered and deployed, the users can begin working with it and sharing their feedback with the developers, helping improve the next software iterations. For instance, once a scalable centralized data repository is complete, the lab staff can immediately start transferring the accumulated research findings from the old databases, structuring them, and removing siloed data.
A crucial part of the QA process for custom ELN software is integration testing, which is needed to prove that laboratory data retains its integrity when being exchanged with laboratory devices, LIMS, LES, and other internal and external systems. Security testing is used to verify software compliance with FDA 21 CFR Part 11, GLP, HIPAA, and other regulations.
5.
Implementation and staff training
After the launch, the development team assists laboratory staff to facilitate the adoption of the new solution. They supply users with written manuals and video guides, give group and individual consultations, and deliver training programs for lab employees.
How Much Does It Cost to Develop Custom Electronic Lab Notebook Software?
The cost of developing an electronic lab notebook ranges from $120,000 to $600,000. The main contributors are the number of systems requiring integration and additional functional modules like an LES or a sample management system.
The table below details the typical feature sets for basic and advanced ELN solutions, including approximate development costs.
|
|
Basic ELN software |
Advanced ELN software |
|---|---|---|
|
Data entry capabilities
|
Free-form note-taking capabilities and an editor for experiment templates, protocols, workflows, and images. |
Specialized document editors (e.g., chemical editor for reaction schemes; molecular biology editor for plasmid sequences) and voice-to-text note-taking. |
|
Communication and data sharing tools
|
|
|
|
Search capabilities
|
Search across texts, tables, graphical elements, and file metadata. |
Search across any data formats (e.g., chemical structures and reaction schemas). |
|
Security and compliance capabilities
?
E.g., RBAC, MFA, version control, audit trails, and e-signatures. |
|
|
|
Integration with internal and external systems
|
1–2 core systems, e.g., LIMS and inventory or asset management systems. |
3+ systems, including data analytics tools, partners’ systems, CTMS, QMS. |
|
Automated data capture
|
Maintaining digital lab logbooks (for equipment calibration and maintenance, quality control, etc.). |
Automated logs for any research and laboratory activities. |
|
Real-time alerts
|
Alerts for compliance deviations and workflow disruptions (e.g., equipment failures). |
Custom alerts for any laboratory events or metrics breaking user-defined thresholds. |
|
Automated test result processing and analysis
|
Rule-based test result processing and test report generation. |
AI/ML-assisted research data analytics (dataset comparison, pattern recognition, etc.) and advanced visualization tools (e.g., interactive data graphs, heatmaps, and 3D molecular structures). |
|
Laboratory execution module
|
Optional |
Optional |
|
Sample management
|
|
Optional |
|
Sample labeling/barcoding
|
|
Optional |
|
Inventory management
|
|
Optional |
|
Cost
|
$120,000-$300,000 |
$300,000-$600,000 |
Why Choose ScienceSoft as Your ELN Development Partner?
- Since 2005 in healthcare IT with 150+ successful projects in the domain.
- Experience in meeting HIPAA, GDPR, FDA, and GxP requirements.
- Expertise in AI and machine learning (since 1989) for laboratory analytics.
- Proficiency in healthcare data exchange standards such as HL7, FHIR, LOINC, and more.
- ISO 9001-certified quality management and ISO 27001-certified information security management.
Our Awards and Recognitions
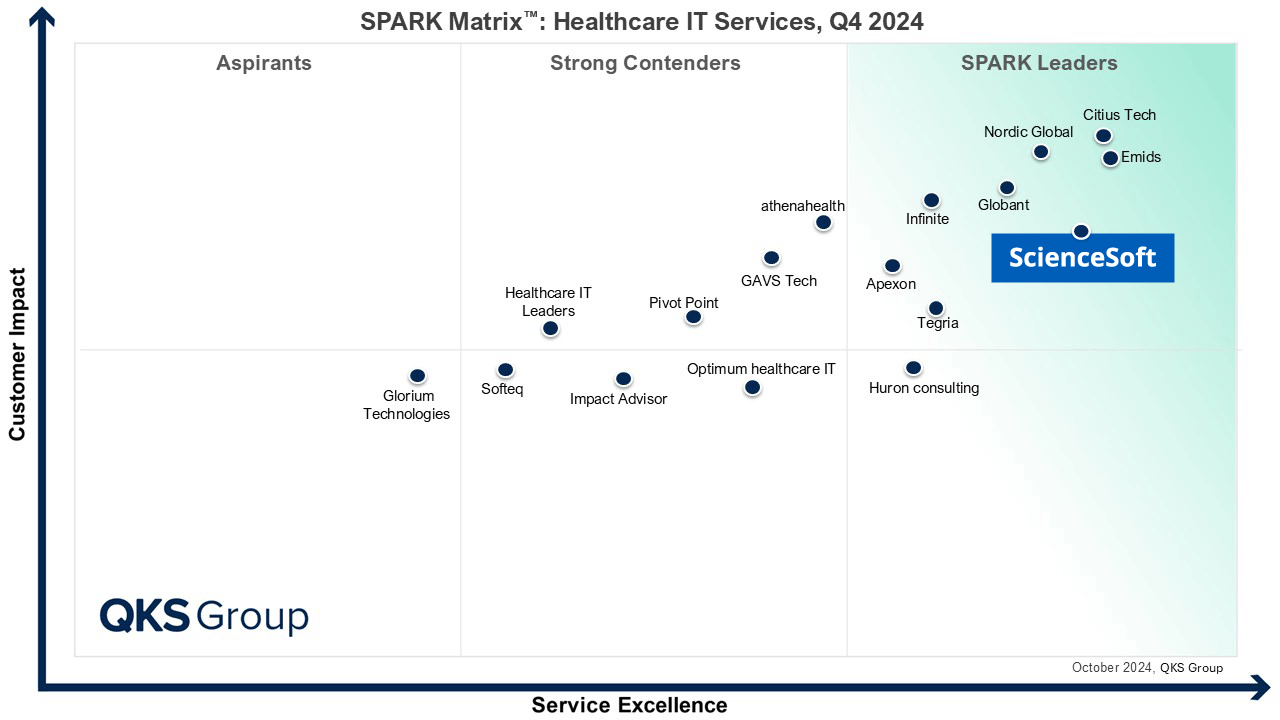
Listed among Healthcare IT Service Leaders in 2022 and 2024
Growing faster than Amazon, Google, and ServiceNow
Recognized for reliability and trustworthiness
Recognized by Health Tech Newspaper awards for the third time

Top Healthcare IT Developer and Advisor by Black Book™ survey 2023
Best in class in medical device connectivity (2023)
A top outsourcing provider for three consecutive years
ISO 13485-certified quality management system
ISO 27001-certified security management system
What makes ScienceSoft different
Driving success in healthcare IT projects no matter what
ScienceSoft develops healthcare IT solutions that reduce care delivery costs and improve outcomes, no matter the challenges posed by diverse expectations of medical staff, shifting priorities, and resistance to change.
