Custom Clinical Trial Management System
Features, Development Process, Costs
ScienceSoft leverages 19 years of experience in healthcare IT to build robust software for efficient clinical trial management.
Clinical Trial Management Software at a Glance
A clinical trial management system (CTMS) is needed to efficiently manage trial data, study protocols, patient recruitment and enrollment, budgeting, study progress tracking, regulatory compliance adherence, and other related workflows.
Custom CTMSs may feature capabilities specific to certain trial domains (e.g., cardiac events prediction for cardiology, automated calculation of Kaplan-Meier curves for oncology). They also have enhanced integration and scalability capacity, which makes them an attractive option for organizations with complex trial portfolios.
- Common integrations for a CTMS: EHR/EMR, HIS, RIS, LIS, ERP, a payment system, a barcode system, a remote patient monitoring system.
- Implementation time: 6 to 18+ months.
- Development costs: $50,000–$500,000+. Use our free calculator to estimate the cost for your case.
Sample Architecture of a Clinical Trial Management System
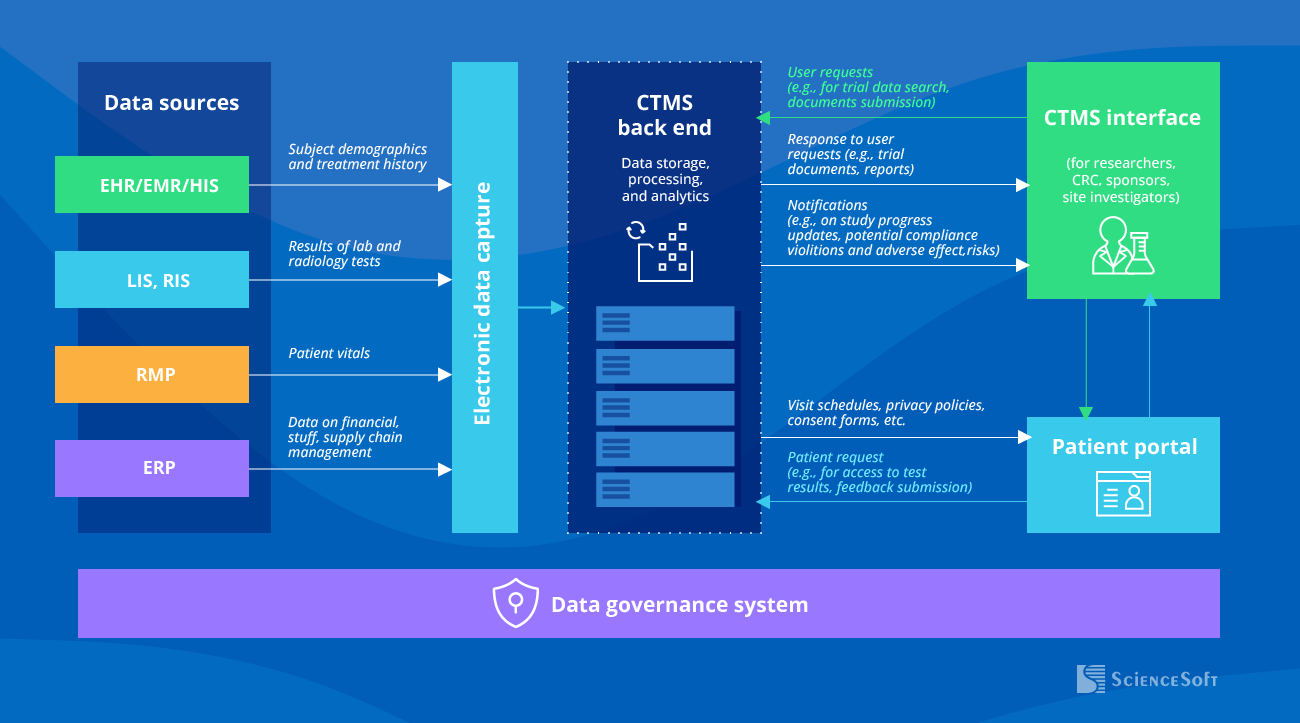
A CTMS can be integrated with a variety of data sources (EHR/EMR, LIS, ERP, and more) for instant access to all the necessary information, including patient data, staff schedules, supplies and equipment availability, finances, and more. A connected electronic data capture system (EDC) is used to streamline clinical trial data collection and management.
The CTMS back end processes and stores the consolidated data in a structured format that is ready for analytics (e.g., in a data warehouse). An analytics engine processes user requests and sends the required information (e.g., data views, scheduled and ad hoc reports, AI output) to the main user interface.
A clinical trial participant portal enhances patient engagement and retention by giving patients instant access to study-related information, schedules, and updates.
The data governance framework includes mechanisms for data backup, encryption, multi-factor authentication, role-based access, data anonymization, and more. These measures are meant to ensure data quality, integrity, security, and privacy in accordance with the relevant regulations such as HIPAA.
Key CTMS Functionality
How to Develop a Clinical Trial Management System
Clinical trial management system software development allows research companies to get a tailored solution for effective management of their study process, sites, subjects, budget, and more. Below, ScienceSoft outlines the key stages of building custom clinical trial management system software:
1.
Requirements engineering
Business analysts conduct interviews with researchers, clinical trial coordinators, sponsors, and other stakeholders to understand the workflows involved in clinical trial management, including data collection, patient recruitment, and regulatory submissions. Based on the acquired information, they define functional and non-functional requirements for the software-to-be, including software capabilities, essential integrations, and security and compliance requirements (e.g., FDA 21 CFR Part 11, HIPAA, GDPR).
2.
Software design
At this stage, software architects decide on architecture components, tools, and techs that would support the workflows defined during business analysis. This can include choosing optimal public-available APIs or developing custom ones to ensure integration with the required systems; deciding on mechanisms for data format standardization and real-time data synchronization. This step also includes elaborating security controls and data management procedures.
3.
UX and UI design
Designers identify role-specific user journeys and build tailored UIs to support their unique workflows. For example, clinical trial coordinators will benefit from a dashboard that prioritizes patient recruitment metrics, visit scheduling, and compliance alerts to optimize the processes of study progress monitoring and participant management.
4.
Development and testing
Arranging development in parallel with testing helps identify and prevent potential issues early, optimize collaboration between development and QA teams, and avoid severe defects in production. Other best practices for optimizing the development process and costs include implementing CI/CD pipelines and DevOps practices, implementing QA automation, and utilizing proven third-party components and microservices (e.g., for data storage components, data management tools). In ScienceSoft’s experience, this approach can help reduce development costs by up to 78%.
5.
Deployment and support
The solution is integrated with the necessary data sources (EHR, LIS, ERP, etc.) and monitored to identify any remaining flaws and promptly implement the necessary improvements (e.g., enhance compatibility between different data formats). Comprehensive software documentation (e.g., configuration and maintenance guides and instructions on API usage) is created to support smooth software deployment, maintenance, and evolution. At ScienceSoft, we also provide one-month post-launch warranty when we fix major software issues free of charge.
What makes ScienceSoft different
Driving success in healthcare IT projects no matter what
ScienceSoft develops healthcare IT solutions that reduce care delivery costs and improve outcomes, no matter the challenges posed by diverse expectations of medical staff, shifting priorities, and resistance to change.
Technologies ScienceSoft Uses for CTMS Development
Clinical Trial Management System Costs
The cost of development for a custom clinical trial management system can vary from $50,000 to $500,000+, depending on software complexity. A custom CTMS can bring a 4-year ROI of up to 270%. The ROI drivers include automation features (e.g., data capture, patient recruitment) that lead to accelerated trial completion, reduced errors and administrative costs. When determining the cost of a custom CTMS, ScienceSoft considers such factors as the number and complexity of integrations, the scope of trial management workflows, the need for real-time data processing and ML/AI-powered capabilities, and more.
$50,000–$150,000
A basic solution that:
- Features core capabilities (e.g., automated electronic data capture, patient enrollment tracking, study planning and progress monitoring, site and resource management).
- Integrates with up to 3 systems (e.g., EMR, ERP, barcode system).
- Offers core analytics capabilities (e.g., historical insights like patient demographics and dropout rates).
$150,000–$500,000+
An advanced solution that:
- Features advanced capabilities (e.g., real-time risk monitoring, built-in payments).
- Integrates with all the required data sources, including legacy and custom systems.
- Offers advanced analytics capabilities, including those powered by ML/AI algorithms (e.g., adverse events prediction, recommendations to optimize resource allocation).
Get a Tailored Quote for Your CTMS Project
Answer a few questions, and our consultants will get back to you with a cost estimate for your case.
Off-the-Shelf vs. Custom Clinical Trial Management Software
There is a wide range of market-available clinical trial management systems that differ in functional scope and complexity, integration capacity, and pricing. None of them is a universal solution, so the preferred tool should be chosen based on the organization’s specific needs. In some cases, custom development will be more feasible. For instance, OOTB solutions may fail to provide sufficient storage and processing capacity for especially data-heavy trials (e.g., genomic research, tracking longitudinal data trends). A custom solution can also be an optimal choice for organizations that carry out multiple trials over extended periods of time: avoiding software subscription fees often leads to substantial cost savings in the long run.
Below, our experts provide a comparison of features and relevant investments for popular market-available software and a custom-built solution.
|
|
Clinevo |
SimpleTrials |
Medidata |
Custom |
|---|---|---|---|---|
|
Basic trial management capabilities
?
Automated electronic data capture, patient enrollment tracking, study planning and progress monitoring, site and resource management |
|
|
|
|
|
Features specific to medical specialties and trial types (e.g., Oncology, Cardiology).
|
|
|
|
Any required specialty-specific features (e.g., disease-specific protocol templates and adverse event reporting tools). |
|
AI-powered capabilities
|
|
|
AI features (pattern recognition and risk prediction) are available upon subscription to a dedicated Medidata product. |
AI/ML capabilities of any complexity, including virtual assistants, medical image analysis, predictive analytics, and more. |
|
Platform
|
Web, iOS, Android |
Web |
Web |
Any (web, iOS, Android) |
|
Ease of use
?
Based on ScienceSoft’s expert assessment. Main criteria: intuitive and customizable UX and UI, short learning curve. |
|
|
|
|
|
Integrations
|
Native: with Clinevo eTMF and Training Management Systems. |
Native integration with 8 EDCs (e.g., Castor, ClinCapture, IBM CD). |
Native integration with Medidata EDC and Site Payments. API integration with third-party EDC, CTMS, eTMF, IxWR, and planning solutions. |
Seamless integration with any required custom and platform-based solutions, including legacy software. |
|
Compliance
|
21 CFR Part 11, EU GMP Annex 11, GxP, and GDPR. |
21 CFR Part 11, SOC 2, PCI DSS. |
HIPAA, GDPR, FDA, CFDA, MHRA, PMDA, SOC 2, PCI DSS. |
Compliance with all required global and regional regulations. |
|
Pricing (50 user accounts / 10 years)
|
Available upon request. |
~$500,000-$600,000 for a plan that covers up to 100 studies and offers basic and advanced capabilities (site and document management, in-built payments, forecasting). NB: There are more affordable options (e.g., up to ~$115,000) with core capabilities and a limited number of users (up to 10). |
Available upon request. |
Up to $500,000+ for an unlimited number of users and studies. No additional fees. |