Custom Laboratory Information System
Features, Architecture, Costs
Since 2005 in healthcare IT, ScienceSoft builds robust, regulatory-compliant systems to optimize the operations of clinical, pathology, public health, veterinary, and other medical labs.
A laboratory information system is needed to manage and automate test and patient-related processes in the laboratory setting, including data entry and exchange, sample tracking, QC assurance, inventory monitoring, equipment maintenance, and more.
Note: Most often, LIS software (laboratory information system) is focused on patient-focused processes, such as test ordering, while a LIMS (laboratory information management system) supports research workflows and has more sample-centered capabilities. However, the borders between the two are not strict, and a LIMS can be used in clinical laboratories.
A custom LIS is usually chosen by organizations that need to cover diverse lab operations (e.g., sample management and equipment management), ensure interoperability with different back-office and external systems, automate the capture of test data from multiple equipment types, and more. A custom solution also allows businesses to benefit from advanced ML/AI-driven features, including automated test result interpretation and what-if modeling.
- Implementation time: 6 to 18+ months.
- Common integrations: EHR/EMR, a healthcare information exchange portal (HIE), a quality management system (QMS), laboratory instruments, molecular diagnostic platforms, Point-of-Care testing devices.
- Costs: $100,000–$800,000+, depending on the solution's complexity. Answer a few questions about your business needs, and our consultants will provide you with a custom quote.
Key Capabilities of a Laboratory Information System
Sample Architecture for a Laboratory Information System
According to Medical Laboratory Observer, nearly half of the surveyed professionals report integration and interoperability issues as the biggest obstacle to automating the processes at their labs.
Below, ScienceSoft’s solution architects provide a sample architecture of an LIS that features sample- and patient-focused workflows and integrates with a variety of enterprise software and external systems for seamless data exchange.
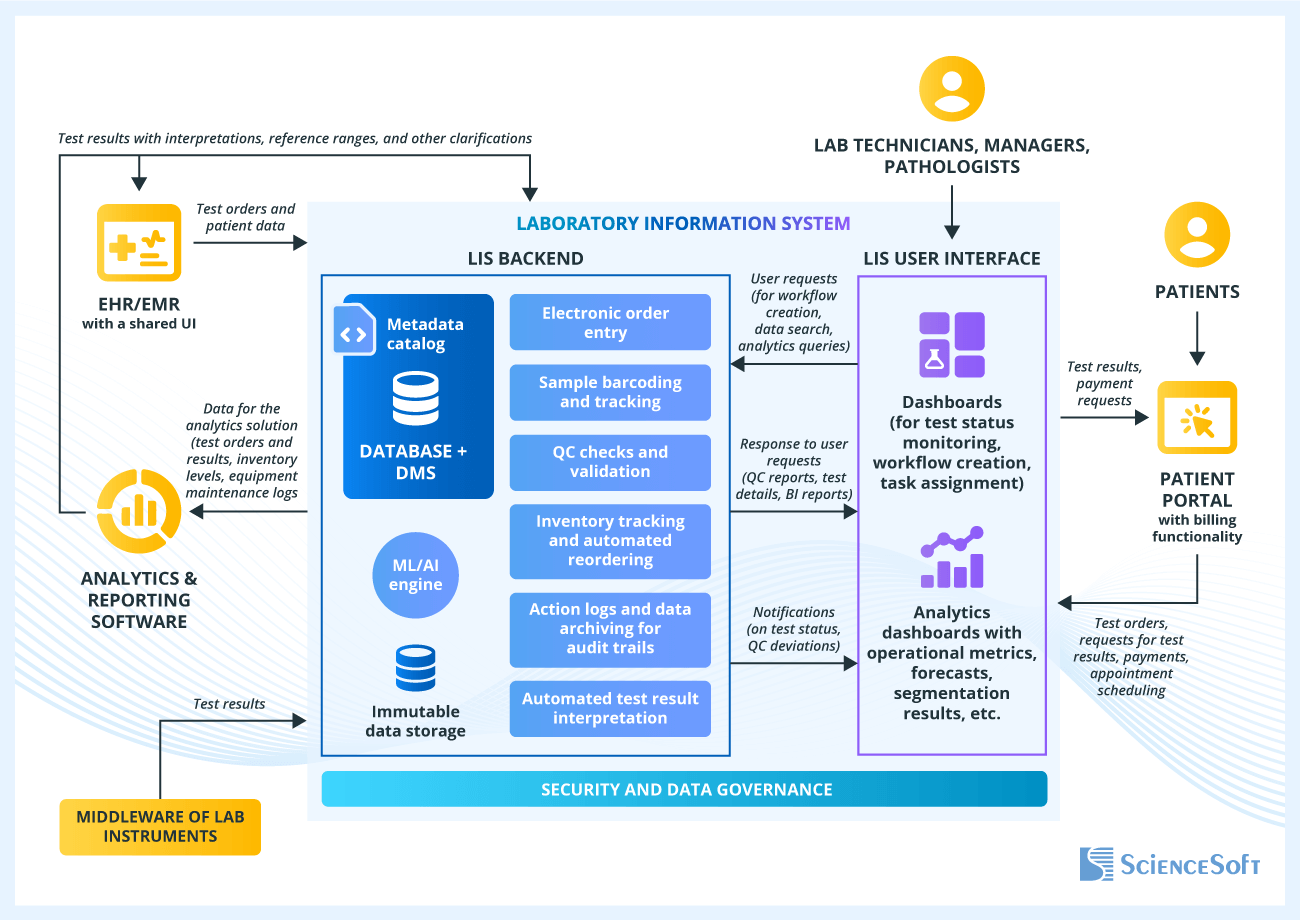
Enterprise software (e.g., EHR/EMR, a practice management system, an HIE portal) is usually integrated via APIs and can have a shared interface with an LIS for submitting test orders and receiving results.
Lab instruments (e.g., HPLC, GC-MS, LC-MS), are often integrated via middleware – an intermediate software layer that unifies data management mechanisms for different instruments.
An LIS is typically integrated with an analytics solution that gathers a variety of lab management data and uses it for calculating lab performance metrics, identifying trends, forecasting and what-if modeling.
LIS backend features an operational database with a database management system and a metadata catalog. The backend processes requests from LIS users (e.g., for sample labeling and registration, workflow creation, quality control setup) and powers all actions and workflows such as sample tracking, test results interpretation, QC checks, inventory reordering, and more. Some actions such as automated test result interpretation can be powered by the ML/AI engine.
LIS user interface provides lab staff with convenient access to tools for receiving test results, tracking lab operations, interacting with the involved parties (e.g., patients, clinicians), and more.
The integrated patient portal with billing functionality streamlines appointment scheduling, test ordering and result delivery, patient-hospital communication, and other related processes.
The data governance and security framework features mechanisms for data masking and encryption, multi-factor authentication, role-based access, data backup and recovery, and more. These measures ensure data integrity, security, and privacy in line with such data protection regulations as HIPAA and GDPR.
Laboratory Information System Development: Key Steps
LIS software development is an optimal choice when an organization needs custom capabilities to cover multiple lab operations, unique sample handling procedures, integration with diverse back-office and external systems, complex analytics features like ML/AI-driven test result interpretation, and more. With 19 years of experience in custom healthcare software development, ScienceSoft provides a brief outline of key steps for successful LIS implementation.
1.
Business analysis and requirement engineering
At this step, business analysts conduct Q&A sessions with the organizations’ management and target LIS users (lab managers, technicians, researchers) to understand their requirements for the LIS. The experts find out what operations the solution should cover, what software the organization uses, and what data exchange processes are to be established between the future LIS and other systems (e.g., test ordering from ERP, test result delivery to a patient portal). The analysts also elicit the types of user roles and their challenges.
At this stage, it is determined what capabilities of the future solution will ensure its compliance with regulations (e.g., HIPAA, CLIA). All the details gathered by business analysts are used to create the list of functional and non-functional software requirements.
2.
Integrations and architecture design
During this stage, solution architects decide on optimal ways to integrate the LIS with the required software and instruments. For instance, to integrate LIS with multiple instruments with differences in their protocols, formats, or interfaces, it is best to opt for middleware that acts as an intermediary between the instruments and LIS. With such an approach, it is possible to centralize data management processes and integrate varied instruments according to the same pattern.
The design team plans the architectural components that will support the outlined workflows and chooses an optimal tech stack to power the solution. The specialists compare suitable techs and pick the ones that can satisfy LIS scalability, availability, and performance requirements at the best cost-to-benefit ratio. When designing the solution’s architecture, the architects also consider the existing IT environment of the organization. For example, if most data sources for integration are Azure-based, the specialists may consider Microsoft tools for LIS development as this will simplify integration and help avoid costs related to purchasing services and products of other providers.
3.
Design of security and data governance frameworks
Security engineers design a comprehensive security and data governance system in line with regulatory and internal requirements. For example, they plan the implementation of multi-factor authentication, role-based user access, and data encryption at rest and in transit, which are crucial for achieving compliance with HIPAA. To ensure complete data accountability and traceability, the experts design mechanisms of audit trail processes. For example, they define events that are to be logged (e.g., record creation, user logins) and choose immutable storage options for audit logs, digital signatures, and other relevant data (e.g., write-once storage, blockchain-based storage). The specialists also plan capabilities for data backup and recovery to prevent data loss and ensure uninterrupted operations in case of emergencies.
4.
UX/UI design
UX designers create solution workflows tailored to specific user roles. For example, quality assurance specialists are likely to benefit from visual alerts on SOP deviations with capabilities for generating the relevant report just by clicking the button in the notification window. For lab managers, the experts may consider no-code capabilities that enable the creation of new workflows by dragging and dropping widgets with procedures, procedure-specific tasks, relevant steps, etc.
UI designers focus on making the solution easy-to-navigate and user-friendly. For instance, they can create color-coded indicators for delays and issues in the sample processing journey. They can also audit the systems the organization already has in use and reuse similar colors, buttons and other familiar visual elements in the LIS to facilitate user adoption.
5.
Development and testing
In most cases, developers and testers work in parallel. This helps promote efficient collaboration between team members and fix arising issues early on.
At ScienceSoft, we always try to find ways to optimize development time and costs and have a set of best practices to achieve this without sacrificing quality. One of such best practices is utilizing cloud services of reputable providers. Thanks to their ready-made components for data storage and low-code development options, they allow for 2-20x faster software development. We often go for DevOps implementation and feasible QA automation, which help us cut development costs for the clients by up to 78%.
6.
Deployment and support
The experts integrate the LIS with the necessary systems and monitor solution performance to detect and fix any remaining issues. They also provide the organization with comprehensive software documentation (e.g., maintenance guides, instructions on API usage) to support smooth software maintenance and evolution.
How Much Does It Cost to Develop a Custom Laboratory Information System?
The cost of custom LIS software development may range from $100,000 to $800,000+. The major cost factors include the scope of LIS capabilities, the need for real-time and ML/AI-powered workflows, and presence of complementary modules like a customer portal and a billing system.
A basic solution
$100,000–$150,000
- Sample and patient management capabilities (e.g., test status tracking, patient profiles).
- Integration with 1-2 core systems like EHR/EMR and HIE.
- KPI monitoring (e.g., turnaround time, billable tests) and BI reporting.
- Real-time capabilities (e.g., for sample status updates).
A solution of medium complexity
$150,000–$300,000
- Inventory management capabilities in addition to sample and patient management.
- Integration with 3-4 systems, including middleware for lab instrument integration.
- Barcode labeling.
- A patient portal for up to 5,000 users.
- Rule-based and ML/AI-powered forecasts (e.g., of turnaround time).
An advanced solution
$300,000–$800,000+
- Covers all lab operations, including equipment, quality management, supply chain management.
- Integration with multiple systems, including QMS and clinical decision support systems.
- A patient portal for more than 5,000 users.
- A billing system.
- ML/AI-powered test result interpretation.
Why Choose ScienceSoft as Your LIS Development Partner?
- Since 2005 in healthcare IT with 150+ successful projects in the field.
- Experience in achieving compliance with HIPAA, GDPR, GCP, and other regulatory standards.
- Since 1989 in AI and machine learning to ensure process automation and complex laboratory analytics.
- Proficiency in healthcare data exchange standards, including HL7, DICOM, ASTM, and more.
- Quality and security management systems backed up by ISO 9001 and ISO 27001 certificates.
Our Awards and Recognitions
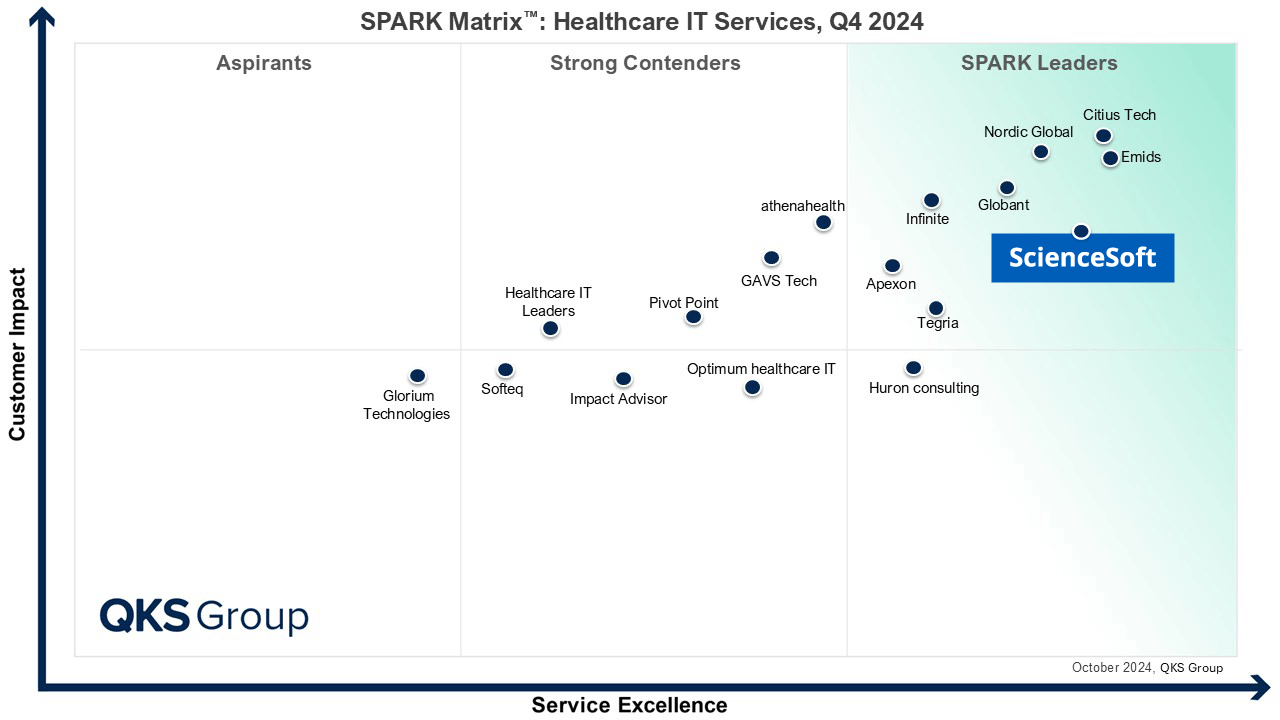
Listed among Healthcare IT Service Leaders in 2022 and 2024
Growing faster than Amazon, Google, and ServiceNow
Recognized for reliability and trustworthiness
Recognized by Health Tech Newspaper awards for the third time

Top Healthcare IT Developer and Advisor by Black Book™ survey 2023
Best in class in medical device connectivity (2023)
A top outsourcing provider for three consecutive years
ISO 13485-certified quality management system
ISO 27001-certified security management system
What makes ScienceSoft different
Driving success in healthcare IT projects no matter what
ScienceSoft develops healthcare IT solutions that reduce care delivery costs and improve outcomes, no matter the challenges posed by diverse expectations of medical staff, shifting priorities, and resistance to change.


